Comprehensive Guide To Methane Lewis Dot Structure And Its Significance
Grasping the Methane Lewis Dot structure is essential for anyone delving into the world of chemistry. This diagram illustrates the arrangement of valence electrons around atoms, offering valuable insights into molecular bonding. Whether you're a student, researcher, or simply someone intrigued by the basics of chemistry, this guide will provide an in-depth exploration of methane's Lewis dot structure and its importance.
Methane, one of the simplest hydrocarbons, plays a pivotal role in both organic chemistry and environmental science. Its molecular formula, CH₄, signifies one carbon atom bonded to four hydrogen atoms. The Lewis dot structure serves as a visual tool to understand how these atoms bond, making it easier to comprehend methane's properties and behavior in various contexts.
In this article, we will delve deeper into the intricacies of the Methane Lewis Dot structure, explore its practical applications, and highlight its importance in the field of chemistry. Whether you're new to the subject or seeking to expand your knowledge, this guide will cover everything you need to know about methane's electron distribution and molecular structure, presented in an engaging and accessible manner.
Exploring the Methane Lewis Dot Structure
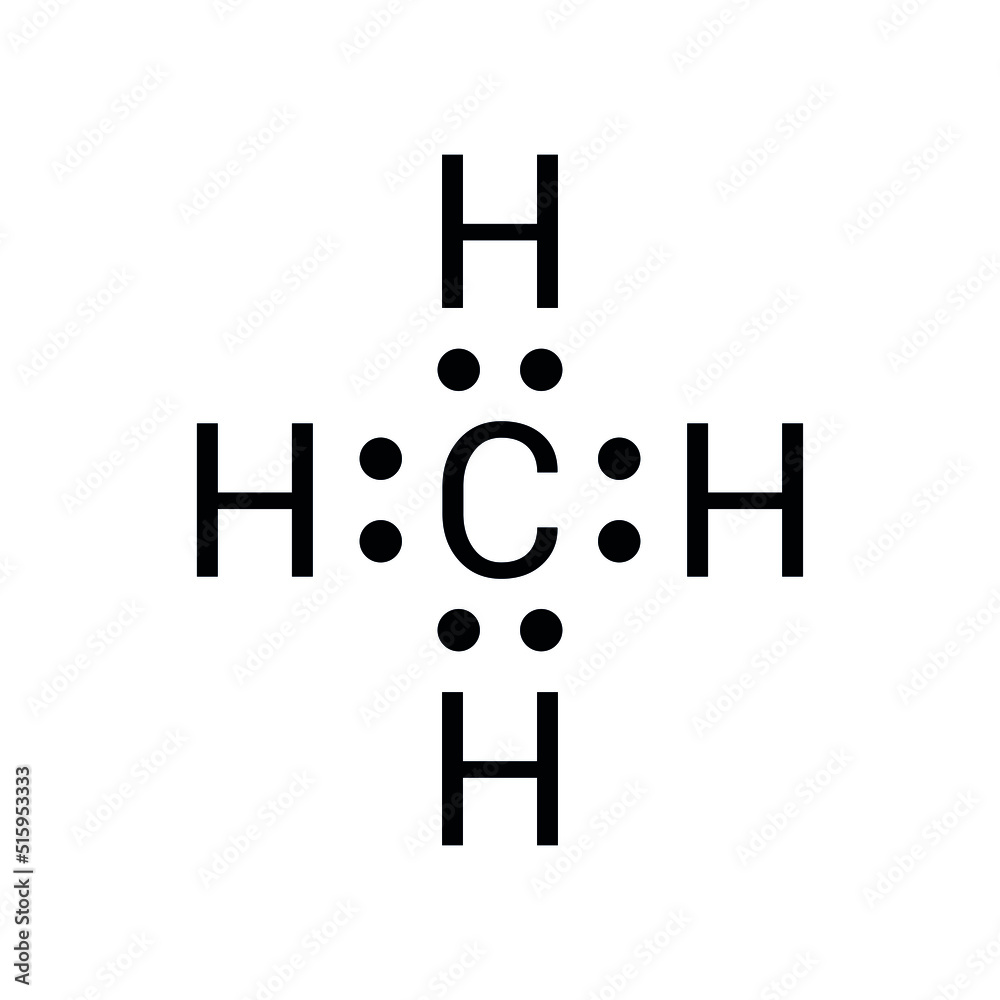
The Methane Lewis Dot structure is a visual representation that demonstrates how valence electrons are distributed among the atoms within a molecule. For methane (CH₄), this structure illustrates the single covalent bonds formed between one carbon atom and four hydrogen atoms. Each bond consists of two shared electrons, ensuring that all atoms achieve a stable electron configuration. This stability is crucial for maintaining the molecule's integrity and functionality.
Methane's Lewis dot structure is relatively simple, as carbon contributes four valence electrons, and each hydrogen atom contributes one. Together, they form a tetrahedral shape, which is fundamental to understanding methane's geometry and reactivity. This geometric arrangement not only defines the molecule's physical properties but also influences its interactions with other substances.
Why Study Methane Lewis Dot?
- To gain a deeper understanding of molecular bonding and electron distribution.
- To accurately predict the shape and properties of methane molecules.
- To apply this foundational knowledge in fields such as organic chemistry, environmental science, and beyond.
Understanding Methane's Chemical Composition
Methane (CH₄) is composed of one carbon atom and four hydrogen atoms. Carbon, positioned in Group 14 of the periodic table, has four valence electrons. Hydrogen, in Group 1, contributes one valence electron per atom. Through covalent bonding, these atoms share electrons equally, forming a stable molecule. This stability is a result of the octet rule, which dictates that atoms tend to gain, lose, or share electrons to achieve a full outer shell of eight electrons. In methane's case, carbon achieves this configuration by forming four covalent bonds with hydrogen atoms, ensuring a balanced and stable structure.
The stability of methane is a testament to the effectiveness of covalent bonding, where the shared electrons create a strong, cohesive molecular structure. This stability is one of the reasons methane is so widely utilized in various industries, from energy production to chemical manufacturing.
Key Features of Methane
- Molecular formula: CH₄
- Valence electrons: 8 (4 from carbon + 1 from each hydrogen)
- Bond type: Covalent
- Molecular geometry: Tetrahedral
Steps to Draw Methane Lewis Dot Structure
Creating the Methane Lewis Dot structure involves a systematic and methodical approach. Follow these steps to accurately represent the molecule:
- Determine the total number of valence electrons: Carbon contributes 4 electrons, and each hydrogen contributes 1, totaling 8 electrons.
- Position the atoms: Place the carbon atom at the center and surround it with four hydrogen atoms.
- Form single covalent bonds: Connect carbon to each hydrogen atom by sharing two electrons per bond.
- Verify stability: Ensure all atoms have a stable electron configuration, with carbon achieving an octet and each hydrogen having two electrons.
Common Mistakes to Avoid
- Forgetting to account for all valence electrons in the structure.
- Misplacing atoms, which can lead to an incorrect representation of the molecule.
- Overlooking the importance of electron sharing in covalent bonds, which is critical for molecular stability.
The Role of Methane in Organic Chemistry
Methane serves as the cornerstone of organic chemistry, being the simplest alkane and a primary component of natural gas. Its stability and reactivity make it an indispensable molecule in numerous chemical reactions, including combustion and substitution reactions. In organic chemistry, methane's Lewis dot structure provides valuable insights into its behavior in different environments. For instance, its tetrahedral geometry significantly influences its interactions with other molecules and its role in energy production.
The simplicity of methane's structure belies its importance in the chemical world. Its properties and behavior are foundational to understanding more complex organic compounds, making it a crucial topic for students and professionals alike.
Applications of Methane
- Energy source: Methane is a key component of natural gas, widely used for heating and electricity generation.
- Industrial applications: It serves as a raw material for producing hydrogen, methanol, and other essential chemicals.
- Environmental impact: Methane is a potent greenhouse gas, contributing significantly to global warming and climate change.
Methane's Molecular Geometry and Bond Angles
The Methane Lewis Dot structure reveals methane's tetrahedral molecular geometry, where the carbon atom is at the center, and the four hydrogen atoms are equidistant from one another. This arrangement results in bond angles of approximately 109.5°, a characteristic feature of sp³ hybridized orbitals. Understanding methane's geometry is crucial for predicting its physical and chemical properties, such as its boiling point, solubility, and reactivity with other substances.
The geometric arrangement of methane's atoms is not merely a theoretical concept; it has practical implications in various scientific and industrial applications. By comprehending the factors that influence methane's geometry, scientists can better predict and control its behavior in different environments.
Factors Influencing Methane's Geometry
- Electron repulsion: The repulsion between electron pairs plays a critical role in determining the molecule's shape.
- Hybridization: Carbon's sp³ hybridization leads to the tetrahedral arrangement, ensuring optimal stability and functionality.
- Environmental factors: External conditions can slightly affect bond angles and geometry, influencing the molecule's behavior in specific contexts.
Comparing Methane with Other Hydrocarbons
While methane is the simplest hydrocarbon, comparing it with other molecules like ethane (C₂H₆) and propane (C₃H₈) highlights the importance of Lewis dot structures in understanding molecular bonding. Each hydrocarbon has a unique structure and set of properties, influenced by the number of carbon atoms and their bonding patterns. For example, ethane's Lewis dot structure shows two carbon atoms connected by a single bond, with each carbon also bonded to three hydrogen atoms. This difference in structure results in variations in reactivity and physical properties, underscoring the significance of molecular geometry in chemistry.
Key Differences Between Methane and Other Hydrocarbons
- Number of carbon atoms: Methane has one carbon atom, whereas ethane and propane have two and three, respectively.
- Bonding patterns: Methane exhibits simple tetrahedral geometry, while larger hydrocarbons possess more complex structures, influencing their behavior and applications.
- Reactivity: Methane is generally less reactive compared to larger hydrocarbons due to its simpler structure, making it more stable under various conditions.
Environmental Impact of Methane
Methane is a significant greenhouse gas, with a global warming potential 28 times greater than carbon dioxide over a 100-year period. Its release into the atmosphere contributes to climate change, making it a critical focus for environmental scientists and policymakers. Understanding methane's Lewis dot structure and molecular properties helps researchers develop strategies to mitigate its environmental impact. For example, capturing methane emissions from landfills and agricultural activities can significantly reduce its contribution to global warming, promoting a more sustainable future.
Efforts to Reduce Methane Emissions
- Improved agricultural practices: Reducing methane emissions from livestock and rice cultivation through innovative techniques.
- Landfill management: Implementing systems to capture methane from decomposing organic waste, turning it into a renewable energy source.
- Energy efficiency: Minimizing methane leaks during natural gas production and transportation through advanced technologies and protocols.
Conclusion
In summary, the Methane Lewis Dot structure provides a foundational understanding of methane's molecular bonding and geometry. By studying this structure, we gain valuable insights into methane's properties, applications, and environmental impact. Whether you're exploring the intricacies of organic chemistry or addressing the challenges of climate change, methane's Lewis dot structure serves as a vital tool for scientific discovery and innovation.
We encourage readers to share their thoughts and questions in the comments section below. For further reading, consider exploring related topics such as the Lewis dot structures of ethane and propane, or delve into the broader implications of methane in environmental science. Together, let's deepen our understanding of the world around us and work towards a more sustainable future.
Table of Contents
Article Recommendations